38 warning labels on drugs
Warnings and Precautions, Contraindications, and Boxed ... This guidance is intended to assist applicants and reviewers in drafting the WARNINGS AND PRECAUTIONS, CONTRAINDICATIONS, and BOXED WARNING sections of labeling, as described in the final rule... FDA Black Box Warnings of 8 Very Common Drugs: Read Your Labels 23.03.2017 · 5. Black Box Warning for Synthroid (Levothyroxine ) (for Hypothyroidism) Synthroid (levothyroxine) is the number one brand-name drug in terms of number of prescriptions written by physicians – over 23 million scrips/month. It is usually given for Hashimoto’s, an autoimmune disease, and when thyroid cancer lowers the circulating thyroxine in the body.
Common and Rare Side Effects for Accutane oral - WebMD COMMON side effects If experienced, these tend to have a Severe expression i ; eye irritation ; an infection of the skin and the tissue below the skin
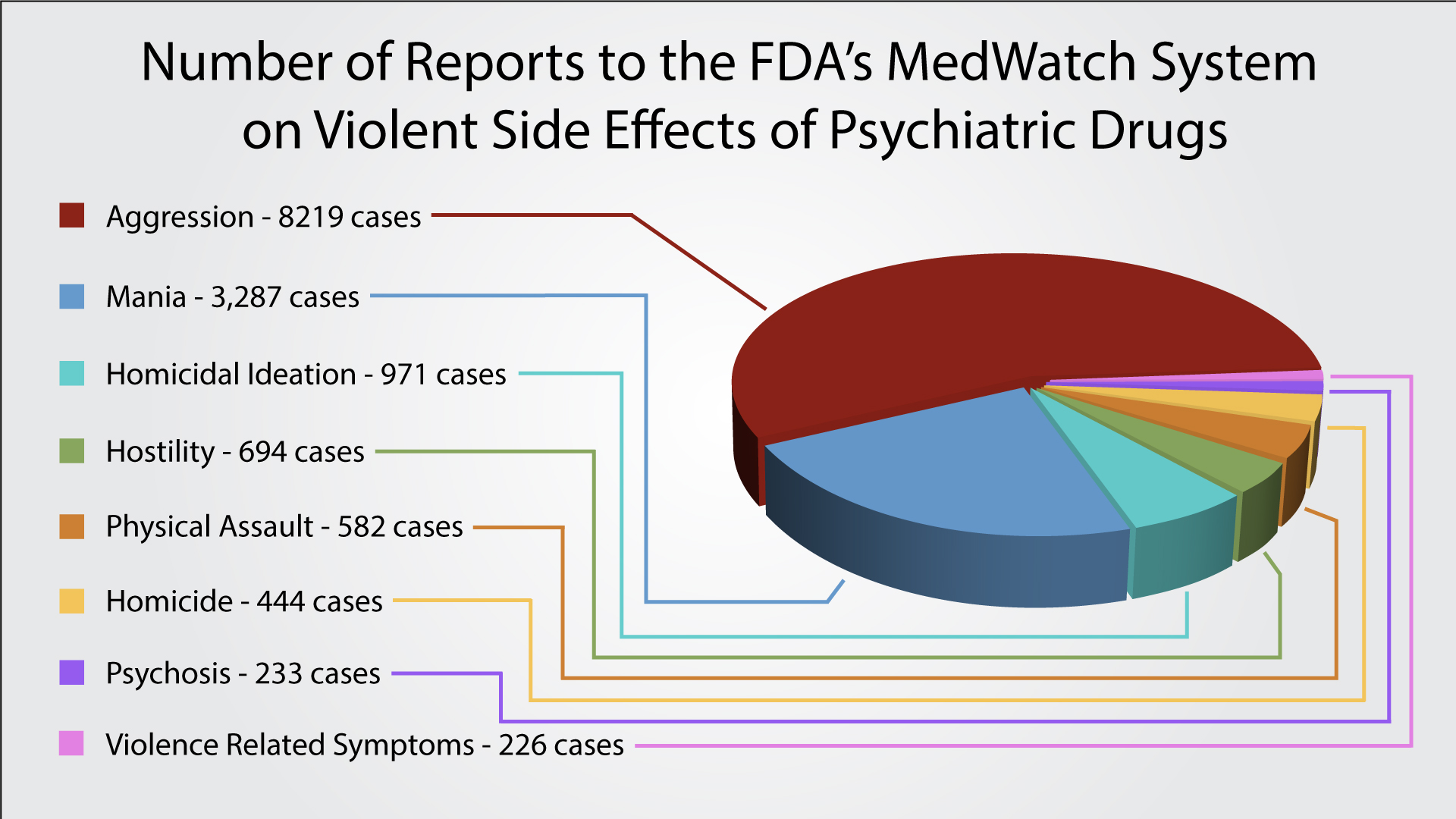
Warning labels on drugs
325 DOT Hazardous Materials Warning Labels and Markings - USPS The warning labels shown in Exhibit 325.3 a, Exhibit 325.3 b, and Exhibit 325.4 may appear only on mailpieces containing mailable hazardous materials that require use of the label under Postal Service requirements. Division 5.1, 5.2, Class 8 and Class 9 labels are only permitted when used in conjunction with a Limited Quantity air mark. FDA Drug Safety Communication: FDA strengthens warning … The U.S. Food and Drug Administration (FDA) is strengthening an existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the … 50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even poisoning.
Warning labels on drugs. Overview of Over-the-Counter Drugs - MSD Manual Consumer Version Often, the labels of OTC drugs do not list the full range of possible side effects. As a result, many people assume that these drugs have few, if any, side effects. For example, the package insert for one analgesic cautions people not to take the drug for more than 10 days for pain. However, the possible serious side effects that can occur with ... Understanding the FDA's Black Box Warnings on Drug Labels Boxed warnings, also known as black box warnings, are the most serious type of warning issued by the Food and Drug Administration (FDA). These warnings are front and center on a drug’s package... Prescription Drug Labels, Understand Directions - Health Discovery An estimated 90 million adults in the United States misunderstand drug labels or have trouble following their directions, according to the Institute of Medicine. Even more troubling is the predicament of many older adults who take several prescription drugs. For them, it’s even more difficult to create a simple schedule for medications with different label instructions. A recent … Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web page
Black Box Drugs | FDA Warning Information 08.12.2017 · According to a 2006 study, nearly 40% of patients in ambulatory settings were prescribed drugs that had a boxed warning. In some of the recorded cases, pregnant women even received medications where the boxed warning made the drug contraindicated in pregnancy. With over 600 medications carrying these severe side effect warnings, it's … Pharmacy, Medication Warning Labels & Stickers | PDC Warning Label "CAUTION: OPIOID Risk of Overdose and Addiction" 1-9/16" x 3/8", Red with White Text, Permanent, 500 per Roll, 2 Rolls per Box. 1-1197. $6.15. Add to Cart. Label Paper Permanent HD Caution: Hazardous Drug 2-3/16"x9/16" Yellow, 1000 per Roll. 1-HD. Boxed warning - Wikipedia In the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears on the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administration specifies that it is formatted with a 'box' or border around the text. The FDA can require a pharmaceutical company to place a boxed warning on the … Cigarette Labeling and Health Warning Requirements | FDA Cigarette Packages. Size and location – The required warning must comprise at least the top 50 percent of the front and rear panels of the cigarette package (i.e., the two largest sides or ...
438 Drug Warning Label Stock Photos - Dreamstime Close-up of a label on a bottle of prescription medication warning not to consume alcohol while using the drug Customer in pharmacy holding medicine bottle. Woman reading the label text about medical information or side effects in drug store. Academic Journals | American Marketing Association Journal of Marketing (JM) develops and disseminates knowledge about real-world marketing questions useful to scholars, educators, managers, policy makers, consumers, and other societal stakeholders around the world. It is the premier outlet for substantive marketing scholarship. Since its founding in 1936, JM has played a significant role in shaping the content and boundaries of … 50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even poisoning. FDA Drug Safety Communication: FDA strengthens warning … The U.S. Food and Drug Administration (FDA) is strengthening an existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the …
325 DOT Hazardous Materials Warning Labels and Markings - USPS The warning labels shown in Exhibit 325.3 a, Exhibit 325.3 b, and Exhibit 325.4 may appear only on mailpieces containing mailable hazardous materials that require use of the label under Postal Service requirements. Division 5.1, 5.2, Class 8 and Class 9 labels are only permitted when used in conjunction with a Limited Quantity air mark.



Post a Comment for "38 warning labels on drugs"